Reimagining Compliance
A Scalable UX Transformation for Enterprize
Platform Thinking I Data Visualization I AI Integration
Key highlights of this project


Project Highlights
Platform Transformation




Data Visualization
AI Integration



Summary
Objective
The Challenge
Lead modernisation of a legacy US FDA
(food & drug administration)
Compliance monitoring platform used by global regulatory consultants
-
Fragmented workflows and inconsistent UI
-
Low user adoption and poor task efficiency
-
Design not aligned with FDA audit standards
Role
-
Set UX vision and strategy aligned to business OKRs
-
Changed mindset from product to platform
-
Created a design system for scalable and compliant UI
-
Evangelized UX across regulatory, product, and engineering teams
Outcome
-
Set UX vision and strategy aligned to business OKRs
-
Improved user efficiency by 30%
-
Reduced onboarding time by 40%
-
Positioned RC platform for FDA audit readiness
-
Elevated UX maturity across the organization
Project Team
A team of 22 members which includes:
Product Manager, Product Designer, Frontend Developer, Backend Developer, Full Stack Developer, QA, Technology Architect, Technology Lead, Scrum Master, DevOps
Duration
-
Ongoing since 2021
Overview
Client

About RegistrarCorp
RegistrarCorp is a U.S.-based regulatory compliance company that assists businesses in complying with U.S. Food and Drug Administration (FDA) and other U.S. Government agency regulations.
Business Vision
Market & Users
“Compliance made easy”
Know sooner, act faster, reduce risk
Helping companies to achieve regulatory compliance by leveraging the registrar's powerful, data-driven, all-in-one solution.
Business Goals
-
Streamline regulatory data accessibility across industries
-
Ensure compliance for all suppliers and shipments entering the United States
-
Provide centralized record management for compliance and audit sensitive assets
-
Market analysis tool for understanding competitors, sourcing qualified suppliers, and understanding industry trends
-
Lower the overall cost for internal regulatory compliance departments
-
Any company regulated by a US government agency
-
Exporter of any FDA-regulated product into the United States
-
Importer of any FDA-regulated product into the United States
-
Facilities that warehouse or store goods
-
Retail importers
-
Foreign governments
-
Shippers
-
Manufacturers
Design Brief
Today’s regulatory service users face a growing challenge: navigating a complex and scattered ecosystem of compliance tools and services.
Despite RegistrarCorp offering a robust portfolio across industries like food & beverage, medical devices, drugs, and cosmetics, users struggle to find, purchase and manage the services they need.
A core area of focus is simplifying how users discover, track, and expand their use of RegistrarCorp services.
In regulatory workflows, clarity and timely action can mean the difference between smooth compliance and costly delays.
Discovery & Analysis
-
Understand the Compliance Ecosystem & Data Gathering
-
Product Understanding
-
UX Audit & Recommendations
-
User Interviews
-
Discovery Workshop With Stakeholders, Product, Legal, Engineering, QA
-
Opportunities, Implications and ROI
-
UX Strategy
-
Modernization Directions
-
Competitor Study
-
Journey Maps
-
Productization Approach
Understand Compliance Ecosystem
Online sessions
-
Stakeholders
-
Product Owner
-
Product Manager
-
Product Documentation Teams
Data Gathering
-
Customer support for service requests
-
Connect with Internal stakeholders, new feature/customization
-
Evaluate product backlog items
-
Connect with Product Management about the product roadmap
-
CSAT & NPS Survey Data

UX Audit & Recommendations
Strengths
-
Risk levels and RegiScore are clearly visible
-
Consistent colour-coded risk indicators
-
The Supplier details panel provides useful information
-
RegiScore visualization is effective
-
Search function available for the supplier list
Recommendations
Replace 'Not Available' with actionable states
• Add filters, sorting, and adjustable pagination
• Standardize status indicators (text + color)
• Confirm destructive actions with dialogs
• Add tooltips and inline help for technical terms
• Improve supplier list readability (grouping, spacing)
Major Issues
Excessive use of 'Not Available' without context
-
Inconsistent status indicators (Low Risk vs No RegiScore)
-
Lack of filters and advanced search options
-
No confirmation for destructive actions (Stop Monitoring)
-
Ambiguous terminology and missing inline help
Summary
The ComplyHub screen is functional and industry-appropriate but can be improved by:
-
Enhancing clarity of system states
-
Improving efficiency with filters and better navigation
-
Reducing clutter by hiding empty fields
-
Providing guidance and documentation for users
Recommendations for Marketplace

Existing Screen
Opportunity Areas
-
Filter UI was changed to user-friendly components to bring consistency in design
-
Details like Product, Compliance and Documents for a Facility were integrated in an accordion for a smooth viewing experience
-
The monitoring button for the facility was made more prominent.

Recommended Design Changes
User Interviews

“I am using few services. Need to pay for all the services”
“For small supplier like me it isn't easy to buy a subscription”
“I am based in the US. I deal with both import and export of goods for multiple industries. For me, it is confusing to see my suppliers and buyers”
“Few services are not online. I need to rely on representatives which is long process”
“The current Dashboard doesn’t give me the information I am looking for”
“Need to go to the RC website for training and other updates about supplier policies”
“I cannot get updates on some of the services I use”
“No UI to add facilities, suppliers, products, News, and other updates for RC”
Goals & Motivations
-
Ensure FDA and other regulatory compliance for clients with minimal delays.
-
Reduce risk of rejected applications and compliance violations.
-
Provide clients with clear, actionable guidance through complex regulatory processes.
Current Workflow
-
Heavy reliance on manual document review and cross-referencing regulations.
-
Use of multiple disconnected tools (spreadsheets, PDFs, email chains) to track compliance steps.
-
Frequent back-and-forth communication with clients for missing or unclear data.
Pain Points
Insights
-
Fragmented systems: Information scattered across tools, no single source of truth.
-
High cognitive load: Need to keep track of changing regulations across industries and geographies.
-
Client delays: Missing documents or incorrect data slow the process.
-
Manual compliance checks: Prone to human error and rework.
-
Manual processes without UI
-
Fragmented services.
-
Need a single source of truth to view insights
-
No customization
-
High cognitive load
-
Delays in documentation
Discovery Workshop: Stakeholders
Identified Areas
Directions
-
Need direction to the user to registration
-
No awareness of FDA registration through RC
-
Improve user registration
-
Option to add facility, supplier, and products
-
Need to redefine subscription plan, service-based
-
User profiling, User Role, Industry
-
How can we use AI to improve productivity
-
Can we automate tasks
-
Current dashboard need to improve to actionable
-
Need an option to see user services
-
Need an option to automate tasks
-
Need a quick solution to fix some issues as it impact user bas
Method
-
Online workshop
-
Post-it notes
-
Dot voting
-
Way for users to discover, buy and continue using RC services
-
Application revamp
-
Build a scalable enterprise application
-
Need a single source of truth to monitor services
-
Build Design system

Workshops with Product, Legal, Engineering, QA
Product
Discussions
-
Roadmap prioritization doesn’t always factor in compliance risk.
-
Complex workflows in the app lead to user confusion and increased support load.
-
Lack of cross-team alignment on compliance-related feature goals.
-
Current UX patterns aren’t optimized for multi-step regulatory tasks.
Opportunities
-
Incorporate compliance impact scoring in feature prioritization.
-
Use workflow simplification as a core design metric.
-
Leverage the design system to standardize compliance-critical interactions.
Legal
Discussins
-
Regulatory interpretation is inconsistent across product teams.
-
Delays occur when legal review happens late in the process.
-
Lack of visibility into upcoming product changes that may require legal oversight.
-
Manual tracking of regulation updates.
Opportunities
-
Embed legal checkpoints early in the design and development process.
-
Develop regulation change alerts integrated into product planning tools.
-
Maintain a central compliance knowledge base accessible to all teams
Insights
-
Compliance impact scoring in feature prioritization
-
Simplify workflows
-
Consideration of the Design system
Insights
-
Legal checkpoints
-
Regulation change alerts in planning
-
Central knowledge base for compliance
Engineering
Discussions
-
Need scalable, reliable architecture
-
Consider cross-platform mobile development
-
Architecture compliant with HIPAA, FedRAMP, SOC 2, GDPR, etc.
-
Consider secure document storage
-
Support for localisation and update translation in real time
-
Figma to Json support
-
Live design system and track how components are used, versioned, and visually tested.
-
Analytics tools such as Mixpanel, Firebase, Hotjar, PowerBi
Opportunities
-
Scalable architecture
-
Security and relevant compliance
-
Development support from design
QA
Discussions
-
Compliance features often fail due to edge-case data issues.
-
Current testing cycles are long and manual, slowing release timelines.
-
Lack of testable mock data for regulatory scenarios.
-
No unified compliance acceptance criteria in product tickets.
Opportunities
-
Introduce compliance test automation for repetitive validation steps.
-
Create a regulatory test data library covering high-risk scenarios.
-
Define compliance QA checklists linked to user stories.
Insights
-
Smooth Design-Dev handoff
-
Analytics tools to track onboarding, retention and user behavior
Insights
-
Legal checkpoints
-
Regulation change alerts in planning
-
Central knowledge base for compliance
Opportunities, Implications and ROI
Opportunities
KPIs to Measure Success
-
Centralized compliance dashboard with case tracking, deadlines, and document status.
-
Embedded compliance rules in forms to prevent invalid submissions. (Evaluate Compliance checks before submission)
-
AI-powered regulation search and real-time updates.
-
Automated reminders for clients to submit required information.
-
Template libraries for recurring compliance document types.
UX & Design Implications
-
Reduction in document rejection rate.
-
Faster case completion times.
-
Lower client support query volume.
-
Higher consultant productivity (cases handled per month).
-
Improved user satisfaction scores from both consultants and clients.
-
Triple-track modernisation:
-
Redesign key workflows while improving usability, engaging and retaining existing user base and building reusable compliance components in the design system.
-
Clear status indicators and progress tracking to reduce client uncertainty.
-
Guided workflows with inline validation for data entry.
-
Mobile-friendly access for consultants working remotely.
-
Analytics dashboards for consultants to track workload, SLA adherence, and client responsiveness.
UX Strategy
Track A: Product Enhancement
Short Term
Engage and retain existing user base
Track B: Product Modernization
Long Term
Track C: Design System
Long Term
User Impact
Compliance users were dealing with legacy UI, inefficient workflows, and avoidable errors. Immediate redesigns could have reduced frustration, time-to-complete, and training costs
User Impact
Platform modernization will lead to less hassle (easy, guided, error-free processes), more control (transparency, self-service, real-time tracking), more trust (secure, reliable, modern experience), more value (insights beyond compliance)
Business Impact
Product enhancement is crutial as it is customer-facing with huge user base of 30,000 clients across 190 countries.
Business Impact
Platform modernization is not just a tech upgrade—it’s a strategic move to stay compliant, enhance customer trust, scale globally, automate manual work, and gain a data-driven competitive edge.
Business Impact
Design system is foundational and strategic. Stakeholders did not see immediate ROI due to no dependency on multiple applications
The design system can accelerate and unify the compliance app and all future products. However, creating a full system first may delay immediate improvements.
• Design systems required developer adoption, governance, version control, documentation, and cultural change.
• Modernising the compliance app needs executive and engineering support. Build credibility and internal alignment via application delivery, then scale system-wide design governance.
User Impact
Platform modernization will lead to less hassle (easy, guided, error-free processes), more control (transparency, self-service, real-time tracking), more trust (secure, reliable, modern experience), more value (insights beyond compliance)
App Modernization – Business Perspective
Evolving Regulatory Environment
-
FDA and global compliance rules keep changing—legacy systems struggle to keep up with frequent updates.
-
Modern platforms allow faster integration of new regulations and automated updates.
-
Ensures businesses stay compliant without costly manual interventions.
Evolving Regulatory Environment
-
Current processes (document management, FDA filings, audits) involve manual effort and disconnected tools.
-
Modernization introduces automation, AI verification, and workflow orchestration, reducing cost and errors.
-
Frees compliance specialists to focus on high-value tasks instead of repetitive work.
Customer Expectations & Experience
-
Current and prospective clients expect intuitive, digital-first experiences like self-service portals, dashboards, and real-time updates.
-
Legacy platforms can feel outdated and reduce trust.
-
Modernization brings user-friendly interfaces, AI-driven chatbots (e.g., Registro), and mobile access for global clients.
Integration & Data Insights
-
Businesses rely on multiple systems (ERP, logistics, suppliers, FDA portals).
-
Modern platforms support API integrations and centralized dashboards.
-
Enables real-time analytics: client trends, risk scores (Regiscore), and performance tracking.
Scalability & Global Expansion
-
RegistrarCorp operates across multiple countries—different rules, languages, currencies, and data security needs.
-
Modern cloud-based platforms (AWS/Azure) enable scalable infrastructure, localization, and multi-language support.
Security & Compliance
-
Regulatory data is highly sensitive. Legacy systems may have vulnerabilities.
-
Modern platforms ensure enterprise-grade cybersecurity, audit trails, GDPR compliance, and FDA data protection.
Competitive Edge
-
Competitors and new entrants are investing in digital-first compliance platforms.
-
Modernization allows RegistrarCorp to differentiate with faster onboarding, predictive compliance insights, and AI-driven support.
-
Helps retain existing clients and attract new ones with a modern digital experience
App Modernization - Users' Perspective
Smoother Onboarding & Registration
-
Today, registering with the FDA is complex and time-consuming.
-
A modern platform offers guided workflows, AI assistance (e.g., Registro bot), pre-filled forms, and progress tracking, reducing stress for new users.
Transparency & Control
-
Users want visibility into where their registration or compliance request stands.
-
Modernization enables real-time dashboards, notifications, and status tracking instead of waiting for emails or manual updates.
Reduced Errors & Faster Processing
-
Compliance errors can mean fines, delays, or rejection by FDA.
-
A modern system can auto-validate documents, flag missing data, and prevent errors before submission.
-
This saves users time, money, and frustration.
24/7 Access & Global Reach
-
Many RegistrarCorp clients are international.
-
A modern cloud-based platform offers multi-language support, mobile access, and self-service options—removing dependency on business hours or time zones.
Trust & Security
-
Users are sharing sensitive regulatory and financial data.
-
Modern platforms give users peace of mind with bank-grade security, audit trails, and compliance certifications—increasing confidence in RegistrarCorp.
Better Insights for Business Growth
-
Users don’t just want compliance—they want to understand risks and opportunities.
-
Modern platforms can provide analytics (e.g., Regiscore trends, competitor benchmarking, market insights) so users make smarter business decisions.
Personalized & Simplified Experience
-
Different users (suppliers, buyers, small businesses vs. enterprises) have different needs.
-
A modern platform can personalize dashboards, recommendations, and reminders based on user profile and industry.
Competitors

FDApals: A regulatory services company with a strong focus on FDA registration and compliance, serving a diverse client base in pharmaceuticals, food, medical devices, cosmetics, and other related industries.

Operon Strategist: A leading medical device regulatory consultant in India, providing turnkey solutions, system implementation, and certifications like FDA 510(k), CE marking, and CDSCO registration.
QPharma: A company specializing in compliance and technology solutions for the life sciences industry.
Medfins International: Specializes in medical device regulatory consulting and helps navigate global market regulations.

National Regulatory Services: Competitor in FDA registration and compliance

The Weinberg Group: Offering services related to FDA regulations.
Existing User Journeys: Importers
-
The platform suffers from poor data relationships, lack of hierarchy, and confusing UX flows.
-
Key actions like editing data, viewing relationships between entities (e.g., products, documents, shipments), and compliance tracking are either missing or not intuitive.
-
Several modules lack contextual information, consistency, and actionable insights.

Existing User Journeys: Exporters
-
Lack of role clarity and user guidance
-
Poor information architecture (hierarchy, navigation, interactions)
-
Missing actionability from key lists and dashboards
-
Inconsistent data relationships across facilities, compliance, and documents

Productization Approach
Services Discovery
-
Awareness about different services (Website)
-
Marketplace
-
Conversion of visitors to registered users (Understand daily page visitors)
User Onboarding
-
User profiling for personalised inputs
-
Self-help for User, Role, Facility, supplier and product management
-
Services Dashboard and recommendations
User Retention
-
Service Customization
-
Subscription
User Registration
-
Frictionless RC registration
-
FDA registration through RC
User Engagement
-
Data visualisation (Unified Dashboard)
-
Industry-Based Dashboards
-
Role-Based Dashboard
-
Dashboard Customisation
-
AI integration
-
Task Automation
-
Supplier Comparison
-
Advance Search
-
Supplier and Product Pages
-
Mobile Application
Governance
-
Add Facilities, Suppliers, Products
-
Review and approval process
-
Content Management (Add, Review, Approve)
-
User, Role, Facility, Supplier and Product management (for specific companies)
Discovery & Research Summary
-
10+ contextual interviews with regulatory consultants
-
Heuristic evaluation of legacy platform
-
Workshops with QA, Legal, and Product
-
Key Insight: 40% time spent navigating fragmented tools
Define and Design
-
Product Roadmap
-
Design Approach
-
Information Architecture
-
Workflows
-
Wireframes
-
Design Options
-
Task Flows and Card Validation
-
Governence
Product Roadmap & Feature Prioritization

Design Approach - Features
Stage: Onboarding
Goal: Help users get to “aha” moment faster
Strategy:
-
In-app tours
-
Smart defaults for document mapping
-
First success tracking
-
Service Awareness through web portal
-
Progressive disclosure of Marketplace
-
Discounted FDA registration
-
Quick onboarding (Without registration Process)
-
FDA registration readiness score for Supplier
-
Personalisation of alerts during onboarding
-
See your Dashboard with quick onboarding
-
Buyer: Upload a supplier document and get an automated compliance check result (Pass/Flag).
-
Consultant: Create a compliance checklist for a client with 1-click AI assistant help.
-
Progressive onboarding (don’t dump all forms at once).
-
Smart defaults (auto-fill addresses, FDA codes, categories).
-
Embedded AI helpers (“Registro” bot explaining why a step matters).
-
Visual progress bar showing “You’re 80% ready for FDA submission!”

Stage: Engagement/Re-engagement
Goal: Pull users back at critical moments and Keep users coming back to take action
Strategy:
-
Contextual alerts
-
Progress dashboards
-
Personalised insights (e.g., “3 facilities at risk”)
-
Email triggers: “Doc expiring”, “New shipment update”
-
In-app nudges
-
Slack/MS Teams alerts (if B2B-integrated)
Deadline-Driven Triggers:
Smart notifications:
-
Automated reminders before FDA renewals, registrations, or updates.
-
30 days before: “Your FDA renewal window opens soon.”
-
7 days before: “Avoid late fees — renew today.”
-
1 day before: “Final chance to submit your documents on time.”
Progress-Based Nudges:
-
Detect incomplete workflows:
-
“You’ve uploaded 3 of 5 documents. Want to finish now?”
-
Personalized re-entry points: deep-link users back to where they left off.
Stage: Retain
Goal: Build habit, demonstrate platform value
Strategy
-
Weekly digest of compliance status (Mails)
-
Compliance score trends (Widgets)
-
Celebrate milestones (“100th doc uploaded!”)
Continuous Value Delivery:
-
Compliance Dashboard: Always updated with status → “You are 92% compliant. Next action: upload GMP certificate.”
-
Predictive Insights: AI flags potential risks (e.g., “Your facility’s FDA registration will expire in 60 days.”).
Habit-Forming Features:
-
Compliance Calendar → central place for all renewal deadlines.
-
Weekly Digest Emails → “Here are your compliance tasks this week.”
Trust + Human Touch:
-
Dedicated compliance consultant assigned to each enterprise customer.
Recognition & Rewards:
-
Certificates of Compliance → easy sharing with buyers & partners.
-
Badges → “Gold Partner – FDA compliant for 3+ years.”
Feedback Loops:
-
After each milestone → trigger a CSAT survey (short & contextual).
Stage: Churn Recovery
Goal: Understand and reduce drop-off reasons
Strategy
-
Targeted survey on inactivity (Survey)
-
Lightweight reactivation flows (Notifications)
-
Incentives like document templates (Templates)
Detect & Segment Inactive Users
Early Signals:
User hasn’t logged in for 30 days, abandoned a form, missed FDA renewal reminders
Guided “Return Path”:
-
When user re-enters app → take them directly to unfinished task.
-
Provide progress indicator: “You already completed 70% of your registration, only 2 steps left.”
-
Offer AI assistant → “Would you like me to finish pre-filling the form for you?”
Trust-Building Through Transparency:
Show clear risk dashboard → “Your compliance score has dropped to 68% due to missing certificate uploads.”
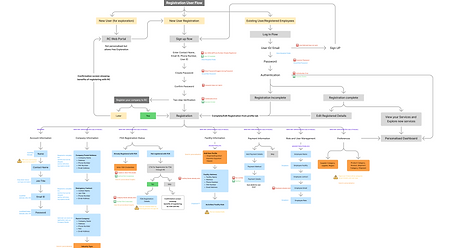
Information Architecture
-
User Registration & Onboarding
-
User Analytics Dashboard (Data Visualisation)
-
User Services Dashboard
-
Compliance Services (Marketplace)
-
Admin

Workflows
Services Discovery & User Registration

User onboarding (Registered User)

User Engagement

Governance

User Retention

Wireframes
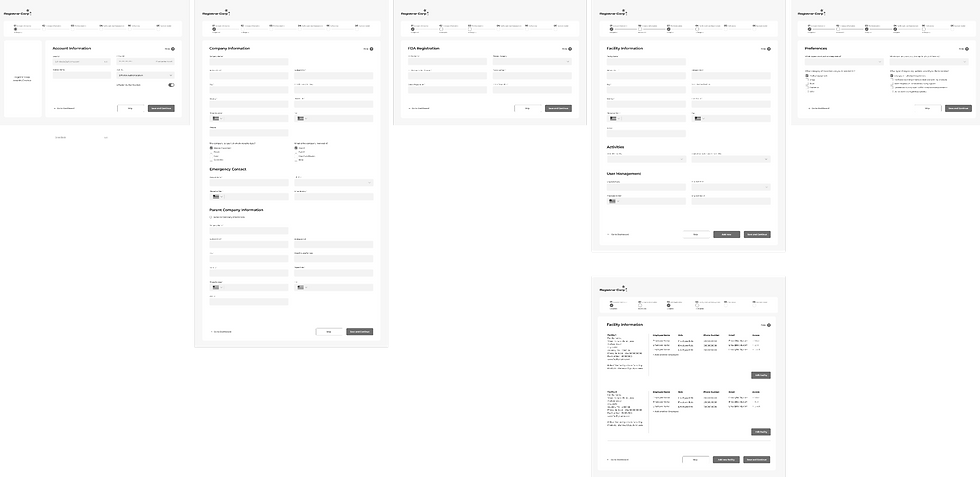
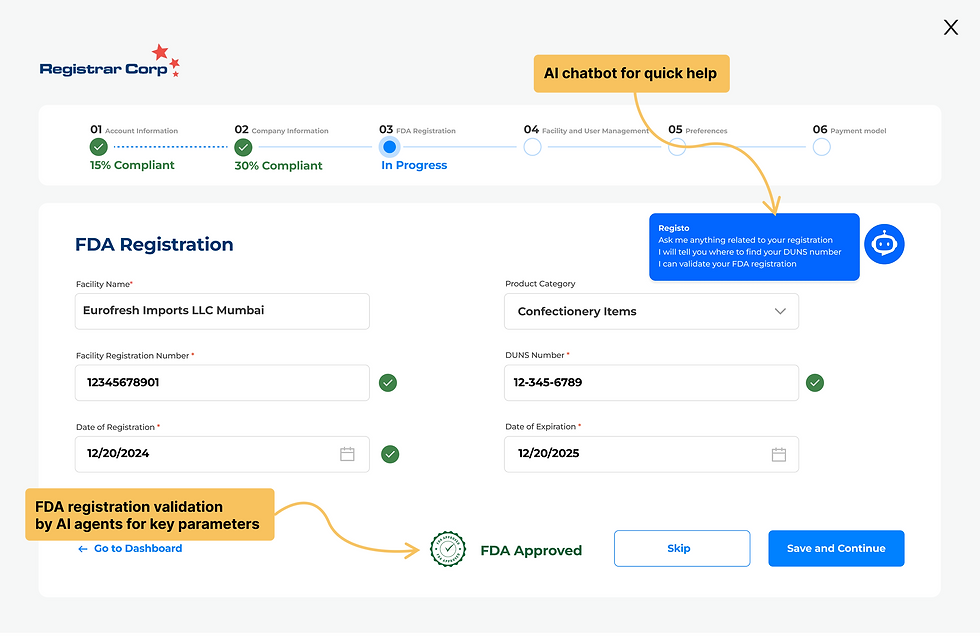
Multiple Design Options
Compliance Dashboard

Option A: Traditional Side Navigation

Option B: Collapsable Side Navigation
Option C: Optimized Side Navigation
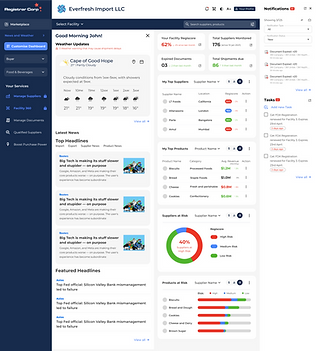
Task Flows & Card Visualization
Task flow for My Top suppliers card visualization and interaction

Governence
Governance dashboard created for RegiistrerCorp Admin

A-B Testing
User Registration Flow

Left Navigation

Top Navigation
Validate
-
Prototype Testing
-
Incremental Design
-
Design Language
-
Technology
-
Localisation
-
Analytics
-
User Behaviour Analysis
-
User Retention
Prototype Testing
Method
Sharing of the prototype by the client with the Buyers, Sellers and taking feedback and doing necessary changes




Incremental Design Changes

Product Release 1

Product Release 3

Product Release 2

Product Release 4
Design Language





Technology

Amazon Web Services
-
Scalable, reliable architecture
-
More services for our use cases than Azure or GCP
-
AWS is commonly used in regulated industries (e.g., health, legal, and compliance).
-
It offers strong compliance certifications: HIPAA, FedRAMP, SOC 2, GDPR, etc.
-
It is also widely adopted among SaaS platforms
-
Popular services for such use cases:
EC2 / ECS : Application hosting
S3: Secure document storage (e.g., compliance documents)
RDS: Relational data storage
CloudTrail / CloudWatch: Logging and monitoring for audits

React Native
-
Build cross-platform mobile Apps
-
Saves time and cost by reusing code as compared to Native iOS/Android development

Hasura Graphql
-
Create role-based schemas and dynamic access control
-
Enables faster front-end development in comparison to REST API

React-js
-
React.js: Modular, scalable, enterprise-grade dashboards and SPAs.
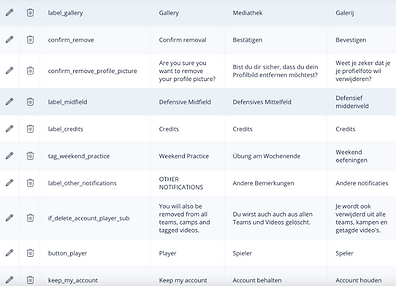
Localization
Objective
-
Globalization and localization of the App.
-
Always have the correct contextual message
-
Update the translations in real-time
-
Keep the messaging powerful at all times
Features
-
Languages: Multi-language UI and documents
-
Regional Compliance: Different flows per country
-
Local Time/Date: Locale-aware date/time formatting
-
Culturally Aware: UI Icons, symbols, colors respectful of culture
-
Role & Region Logic: Region-specific access to features and content
Accessibility
Section 508 (U.S. Federal Requirement)
-
Requires electronic and IT systems procured/used by U.S. government to be accessible.
-
Maps closely to WCAG 2.0 AA standards.
ADA (Americans with Disabilities Act)
-
U.S. law requiring digital products (including SaaS apps) to provide equal access.
-
Especially relevant if customers include U.S. businesses, medical device manufacturers, or importers/exporters.
EN 301 549 (European Union)
-
EU’s accessibility standard for ICT products and services.
-
Based on WCAG 2.1 AA.
Global Variations
-
Canada: Accessible Canada Act (ACA).
-
UK: Equality Act 2010 + Public Sector Accessibility Regulations.
-
Australia: Disability Discrimination Act (DDA).
-
India: Rights of Persons with Disabilities Act (RPWD).
-
App Should IncludeText alternatives: Alt text for all non-text content.
-
Keyboard-friendly design: All workflows (forms, document uploads) must be operable without a mouse.
-
High contrast modes for dashboards and regulatory forms.
-
Screen reader support (NVDA, JAWS, VoiceOver).
-
Error prevention in compliance forms: Clear validation, confirmation before submission.
-
Accessible PDFs / documents (important since FDA filings often involve downloadable forms).
-
Multilingual accessibility: Support for translations, right-to-left scripts if targeting global clients.
Accessibility Features RegistrarCorp
Analytics
Understand how users interact with the product and identify friction.
Ensure visibility into how well core compliance processes are running.
-
Task completion rate (e.g., “Create Shipment” flow)
-
Drop-off points in onboarding or compliance processes.
-
Time spent in non-functional areas (indicates confusion)
-
NPS/feedback widget integration points

Mixpanel
-
Feature usage, paths, drop-offs (e.g., % of users who upload documents but don’t link them).
-
Event-based analytics with Mixpanel
-
Aids to draw user journeys and actionable insights
Design System
-
Tag components in live apps to measure real-world adoption (e.g., how often “Regiscore” widget is used).
-
% of apps using only DS-compliant components
-
Most/least used components
-
Time to integrate a new component
-
Component version drift across modules
Branch
Integrated to attribute conversions to marketing campaigns

Storybook
Track how components are used, versioned, and visually tested.

Firebase
-
For crash analytics
-
For A/B testing features and measuring feedback
User Behaviour Analysis

User Retention

Project Management
-
Productization Process
-
Collaboration Model
Productization Process

Collaboration Model

Outcome, Learnings and Impact
-
Workflow and Design Highlights
-
Design System
-
Collaboration and Change Management
-
Measurable Outcome
-
Organizational Impact
-
Reflections & Learnings
Workflow & Design Highlights
-
Introduced task-based dashboard
-
Consolidated 8-step process into 3-phase flow
-
Context-aware alerts and regulatory flags
Collaboration & Change Management
-
30+ Weekly design showcases to execs
-
Aligned with SMEs on compliance semantics
-
Coached internal design team on system thinking
Design System
-
150+ components, tokens, templates
-
Compliance-ready UI (audit logs, e-signatures)
-
Governance model with Product & Engineering
Outcome
-
First internal UX Guild launched post-project
-
Design System adopted by 2 more product lines
-
UX team gained strategic seat in planning forums

Organizational Impact
-
First internal UX Guild launched post-project
-
Design System adopted by 2 more product lines
-
UX team gained strategic seat in planning forums
Reflections & Learning
-
Compliance UX needs clarity, traceability, and confidence
-
Scalable design requires governance, not just templates
-
Design influence grows through business alignment
